

this is a strong, narrow trough at 1680-1750 cm-1 in aldehydes, ketones. Note that the O=C stretch of the alpha, beta-unsaturated compound - benzaldehyde - is at a lower wavenumber than that of the saturated butyraldehyde. The IR spectrum is a measure of the transmittance of IR against the wavenumber. For one thing, all of these functional groups appear to the right of the C-H absorptions, which always occur between 2,800 cm 1 to 3,000 cm 1 in the IR spectrum, and. The spectra of benzaldehyde and butyraldehyde are shown below. You can locate carbonyl groups, alkenes, alkynes, and aromatics in the IR (infrared) spectrum, based on their shapes and relative locations.

This band generally appears as one or two bands of moderate intensity in the region 2830-2695 cm -1. See also:Īnother useful diagnostic band for aldehydes is the O= CH stretch.
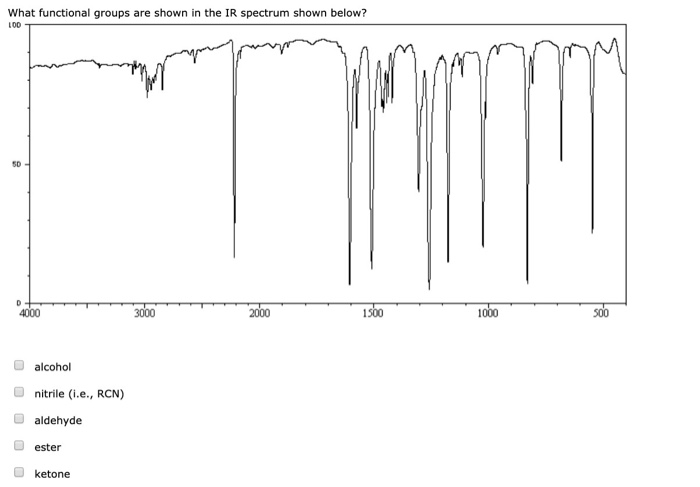
As in ketones, if the carbons adjacent to the aldehyde group are unsaturated, this vibration is shifted to lower wavenumbers, 1710-1685 cm -1. The carbonyl stretch C=O of saturated aliphatic aldehydes appears from 1740-1720 cm -1. IR: aldehydes IR Spectroscopy Tutorial: Aldehydes


 0 kommentar(er)
0 kommentar(er)
